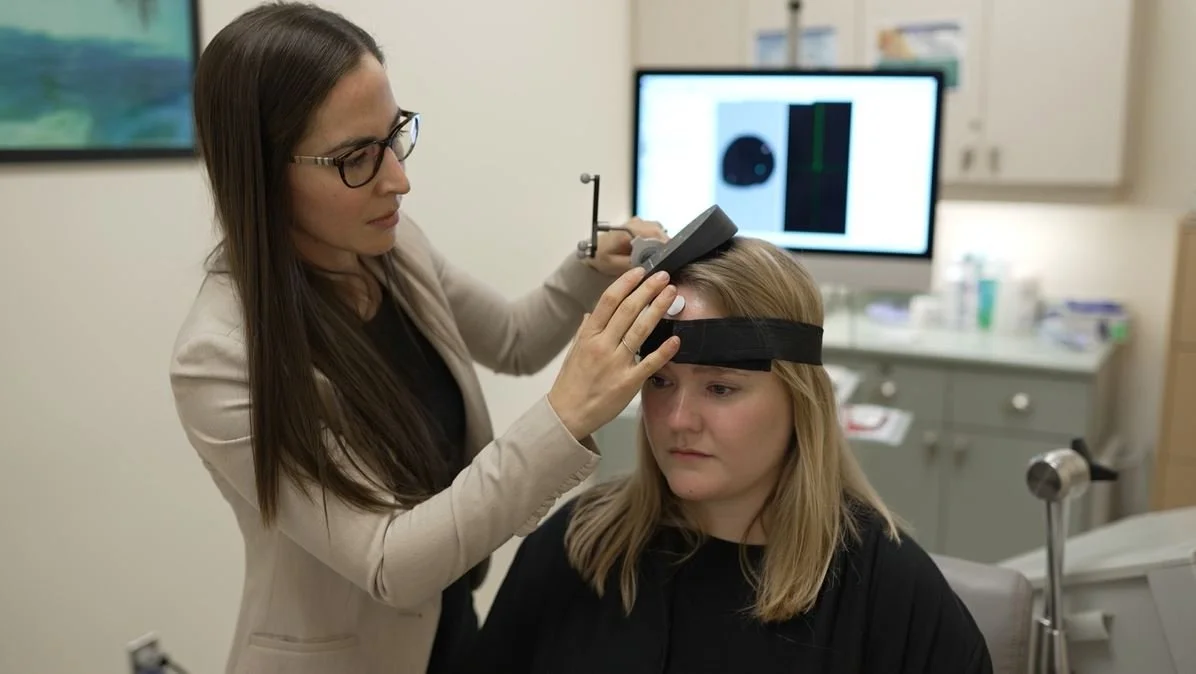
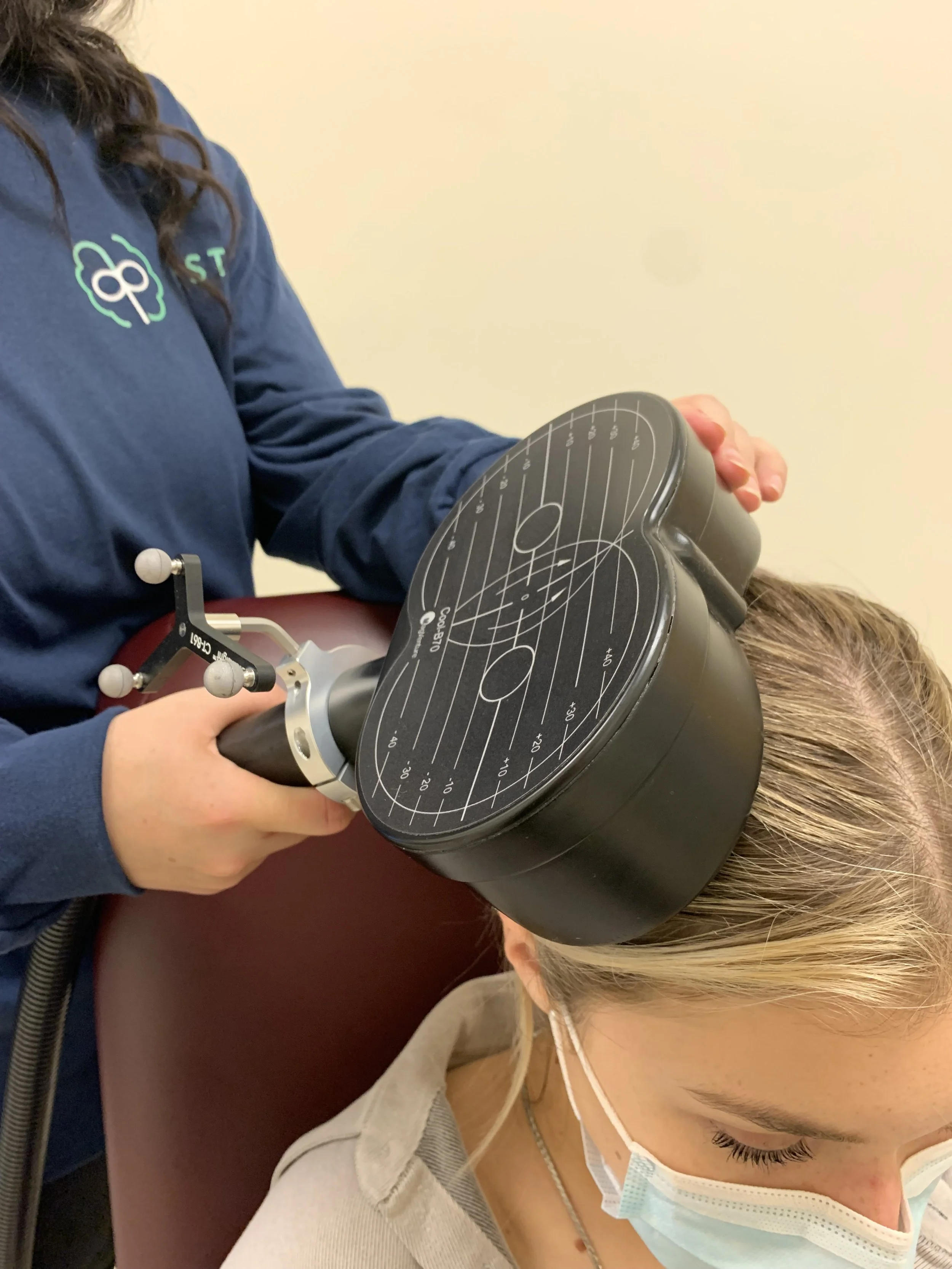
Current Research Projects
A naturalistic study comparing the efficacy of uni- and bi-lateral theta burst stimulation in treating major depression
This study is the largest project in our lab! The main goal of this study is to find the optimal treatment for people with treatment resistant depression using a type of transcranial magnetic stimulation (TMS), called theta burst stimulation (TBS). We also hope to find neurobiological mechanisms that can predict who will and won’t respond to this type of treatment.
Study Design:
An individual can be referred by their family doctor or psychiatrist if they have a diagnosis of major depressive disorder (MDD). Once we have received the referral the individual will participate in a telephone screening to assess them for baseline eligibility. If they are eligible the individual will attend three clinical visits before the start of treatment to further confirm their eligibility. During these visits, individuals will be asked to sign a consent form, complete a clinical interview, self-report questionnaires, TMS-related measurements, a urine sample, and a have a consultation with the study psychiatrist. Once everything is in order, the individual will begin the treatment phase of the study.
To begin the individual will be booked for an MRI, Calibration, and an EEG session. Following these sessions the individual will be receiving treatment five days a week (Monday-Friday) for 4-6 weeks. Each daily TMS session takes about 20-30 minutes. Following treatment the individual will have another EEG session followed by another clinical visit where they are assessed. If individuals do not respond to treatment after the sixth week, their participation in the study will end and they will be discharged back to their referring doctor. If individuals do respond and go into remission after the sixth week, they will be invited to move onto the maintenance phase. The maintenance phase is completed over the course of six months, where individuals will receive TMS treatment. Once maintenance is complete and the final assessments have been completed, individuals will be discharged back to their referring doctor thus ending their participation in the study.
OBJECTIVES:
To compare the efficacy of bilateral and unilateral TBS on symptoms of depression, as well as rates of remission and response.
To investigate how unilateral and bilateral TBS modulates brain activity in the dorsolateral prefrontal cortex (DLPFC) using interleaved TMS and electroencephalography (TMS-EEG) by measuring changes in levels of cortical inhibition and excitation.
To investigate if baseline capacity for plasticity and inhibition are predictive of the clinical response to TBS.
To compare the efficacy of a fixed versus a flexible schedule of maintenance on a period of 6 months on symptoms of depression and rate of relapse.
Neural mechanisms and predictors of response to electroconvulsive therapy: a transdiagnostic approach
This study uses a different neuromodulation technique called electroconvulsive therapy (ECT). Our labs main goal is to determine a possible predictor of response to treatment because ECT currently has only a 60-70% response rate. We are looking for a possible predictor to better understand why some people respond to treatment and some people don’t. We are also exploring the neural mechanisms, clinical and cognitive effects of ECT.
Study Design:
To participate in this study an individual must have a diagnosis of depression or schizophrenia and have been referred for ECT treatment at the Royal Ottawa Mental Health Centre. Once an individual’s referral form is received by their physician, a telephone screening will be conducted to assess for study eligibility. If the individual is eligible, they will come to The Royal three times for study visits; once before ECT treatment and twice after. The first visit will be 72 hours before the first ECT treatment and will consist of signing a consent form, clinical and cognitive assessments, and TMS-electromyography (TMS-EMG) recordings. The second will be within 72 hours after the last acute ECT treatment and will consist of the same clinical, cognitive and TMS-EMG assessments as visit one. The last visit will be one month after the last acute ECT treatment and will consist of the clinical and cognitive assessments. Investigators on this project have no involvement in the treatment of ECT.
OBJECTIVES:
To determine the presence of a neurobiological predictor of clinical response to electroconvulsive therapy (ECT) in mental health disorders using interleaved transcranial magnetic stimulation and electroencephalography (TMS-EEG), specifically measuring levels of cortical inhibition and excitation in the motor cortex.
To investigate the neural mechanisms of ECT using interleaved TMS-EEG, specifically by measuring changes in levels of cortical inhibition and excitation in the motor cortex.
To monitor the effects of ECT on global cognition and autobiographical memory using the ElectroConvulsive therapy Cognitive Assessment (ECCA)
A placebo-controlled study comparing the effects of unilateral and bilateral theta burst stimulation on prefrontal activity
This study is a control study where we look at the effects of TMS on both sides of the brain compared to one side of the brain. This study is working with individuals who are not diagnosed with a mental illness. Having a control study like this one is can help us understand the effects of TMS on the brain and better understand the brain of people living with a mental health diagnosis.
OBJECTIVES:
To compare the effect of unilateral theta bust stimulation (TBS) and bilateral TBS on dorsolateral prefrontal cortex (DLPFC) activity using three transcranial magnetic stimulation and electroencephalography (TMS-EEG) measures:
a) TMS-evoked potentials reflecting local cortical excitability
b) TMS-evoked oscillations
c) Connectivity between prefrontal regions and related networks
To explore if baseline TMS measures of plasticity and inhibition in the primary motor cortex can predict response to TBS applied to the DLPFC.
Study Design:
This study involves individuals with no mental health diagnoses and therefore does not require a referral form from a doctor. The first step is for individuals to complete a telephone screening to assess for eligibility and obtain consent. Following this, there will be an in person MRI session. Finally there will be three testing visits. The first visit will include a clinical assessment and TMS measurements. Visits 3 and 4 are identical with the exception of the stimulation condition. During these visits a clinical assessment will take place, as well an EEG, and stimulation. Each session will last approximately 2-3 hours. Total duration is approximately 7.5 hours over three weeks.
Neural mechanisms of intermittent theta burst stimulation on the core depression network
This study is trying to improve our understanding of rTMS with the help of brain imaging techniques such as positron emission tomography (PET) and magnetic resonance imaging (MRI). This study aims to help us determine what factors make this treatment effective.
OBJECTIVES:
To determine if intermittent theta burst stimulation (iTBS) decreases subgenual anterior cingulate cortex (sgACC) metabolic activity in individuals with Major depressive disorder
Second we want to determine whether iTBS induced decrease in metabolic activity can be predicted by connectivity measures
Investigate whether the. magnitude of decreased sgACC metabolic activity after iTBS can predict response to a 4-week treatment course of iTBS
Study Design:
An individual must be referred by their family doctor or psychiatrist (not acceptable from a therapist, psychologist, counsellor, etc…must be an MD) and have a diagnosis of major depressive disorder (MDD). Once the referral is received, we will conduct a telephone screening to assess for baseline eligibility. If an individual is deemed eligible, they will be put on the wait list until an available spot opens up. Individuals must attend three clinical visits prior to the treatment start date to confirm eligibility. During these visits, individuals must sign a consent form, complete a clinical interview, self-report questionnaires, TMS-related measurements, a urine sample, and a have a consultation with the study psychiatrist. Once everything is in order, the individual will begin the treatment phase of the study. This includes full time TMS treatment which happens Monday-Friday for approx. 20-30 minutes daily for 4weeks. Individuals will also have to undergo two PET scans one week apart prior to the first treatment session. Once the final assessments have been completed, individuals will be discharged back to their referring doctor thus ending their participation in the study.